LSG: thermoplastics shapes for life science applications
The Life Science Grades (LSG) are a suite of thermoplastic shapes. Intended for use in short-term body-contact applications in the medical field as well as bioprocessing applications, LSG materials are pre-assessed for biocompatibility both at the resin and stock shape levels.
These plastic shapes meet stringent requirements for material performance, pre-assessment testing, and risk management:
- Broad portfolio of materials made from first-class medical resin suppliers - The Life Science Grade stock shapes encompass a comprehensive range of polymer types (HDPE, PP, PC, PSU, PEI, PPSU, and PEEK) and are produced from only the highest quality resins that are pre-assessed for biocompatibility.
- Full traceability and quality assurance - Materials undergo additional biocompatibility pre-assessments at the stock shape level, and the production and machining of components is undertaken within ISO 13485 quality management systems.
- Expert support throughout - From material selection to compliance topics to processing know-how, we support you in validating your approach every step of the way.
-
When selecting a Life Science Grade material for your project, we recommend you start by gathering the specific requirements of your application: both in terms of compliance and performance.
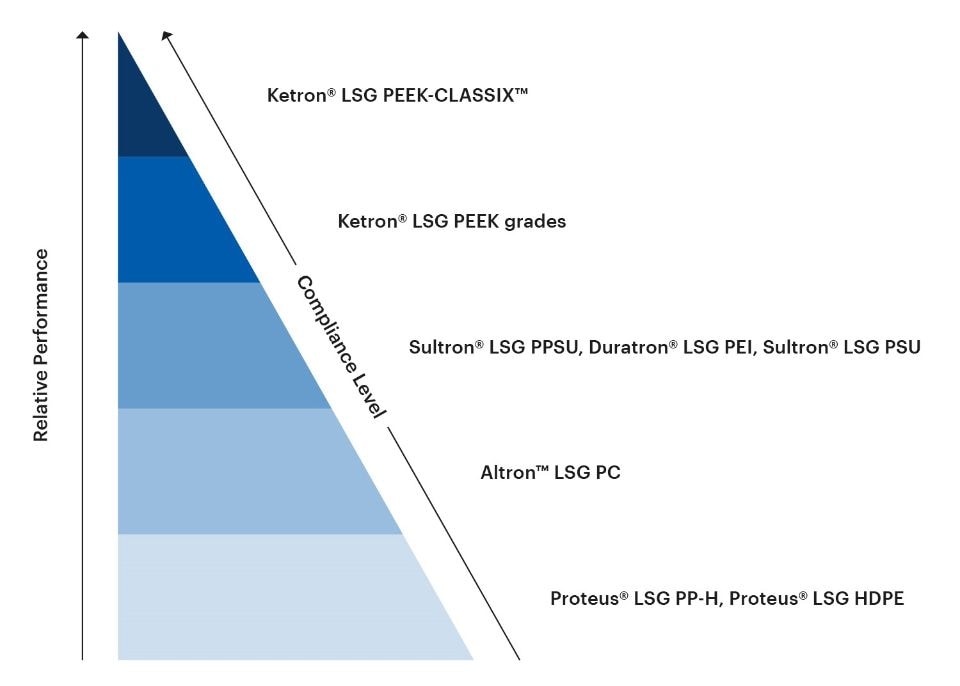
The LSG portfolio of thermoplastic shapes encompasses a wide range of materials with different levels of performance properties relevant to medical and bioprocessing applications, including stiffness, temperature resistance, dimensional stability, impact strength, sterilizability, chemical resistance, and gamma radiation resistance. The LSG portfolio also spans an array of different compliance levels for different intended uses in the medical and bioprocessing fields.
The following chart gives an at-a-glance overview of the relative performance and compliance levels of the LSG portfolio.
-
The Life Science Grades are resilient against many chemicals commonly found in healthcare applications. Review the plastic/chemical compatibility chart below as a rough guideline to understanding which thermoplastic material will resist the chemicals relevant to your application.
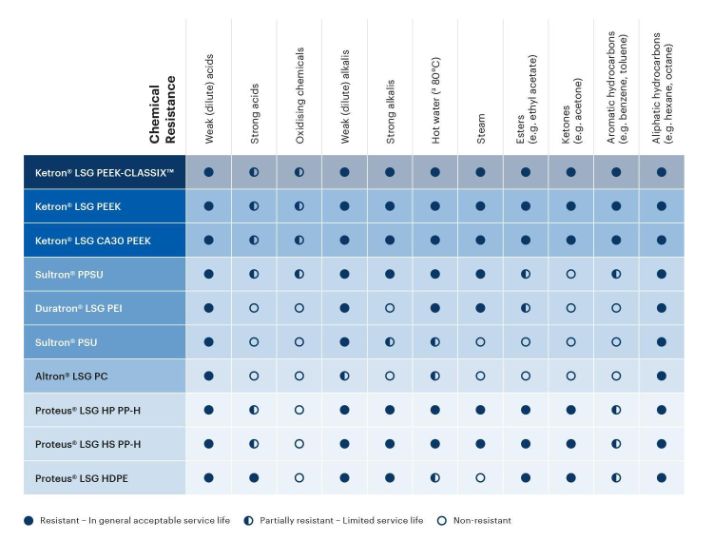
Is there another chemical present in your application? More detailed chemical resistance information can be found in our full database of thermoplastic chemical compatibility.
-
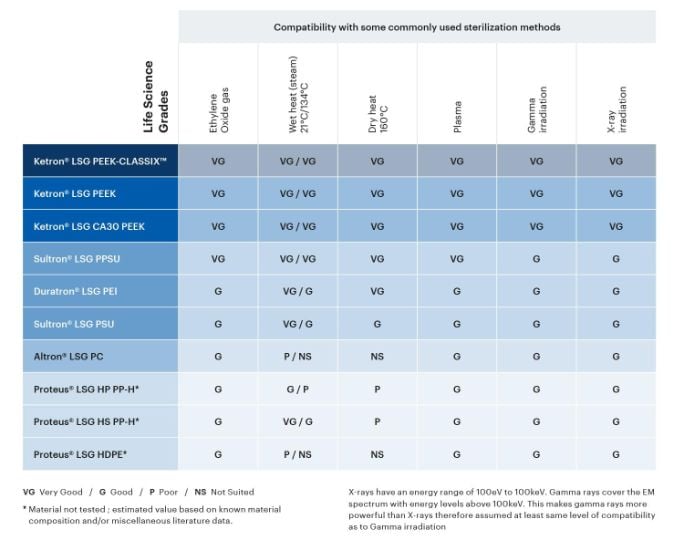
Various sterilization methods are used in the medical industry to kill all viable micro-organisms on a part or device before use. Given their intended use in applications involving direct or indirect contact with human body tissue, the Life Science Grades were designed to be compatible with modern sterilization techniques such as EtO (ethylene oxide gas), autoclave (steam), dry heat, plasma, and irradiation by gamma or X-ray.
Explore the sterilization compatibility table below to evaluate which thermoplastic materials may be good candidates for your application.
-
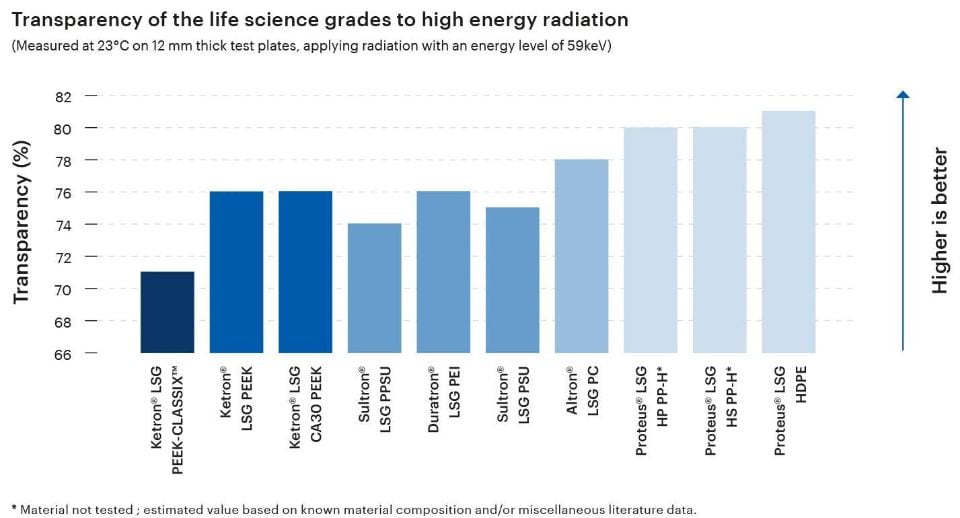
The transparency of a plastic material to high-energy radiation is a highly relevant attribute in the material selection process for medical applications, especially those involving medical imaging or radiation therapy. Thermoplastic materials used in these applications must allow x-rays and/or gamma rays to pass through them without significant attenuation in order to enable clarity and accuracy.
The Life Science Grades from Mitsubishi Chemical Group exhibit transparency levels between 71% and 81%, making them excellent candidates for components in the fields of medical imaging, radiation therapy, or dosimetry.
-
The level of resistance exhibited by a plastic material to gamma radiation is a crucial consideration in medical, life science, and bioprocessing applications. Adequate gamma radiation resistance helps to ensure effective sterilization, maintain product integrity and safety, and the support long-term performance of components and devices.
Given their intended use in medical, life science, and bioprocesing applications, the Life Science Grades from Mitsubishi Chemical Group are designed to exhibit high levels of resistance to gamma radiation.
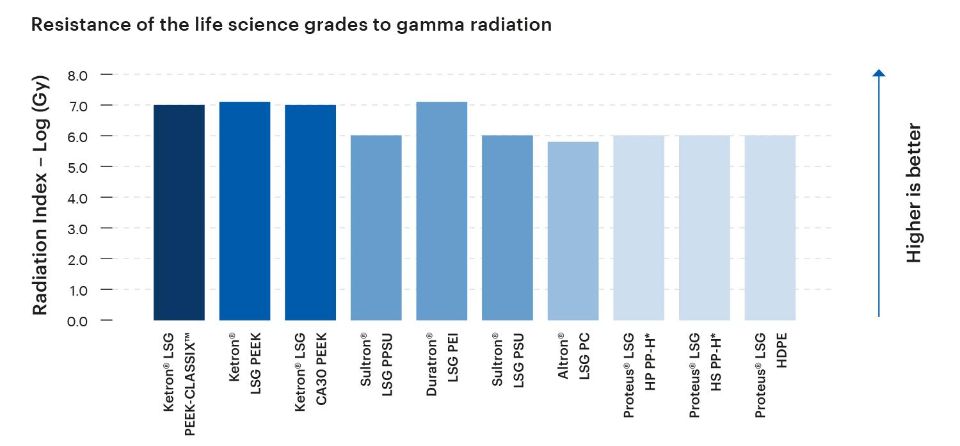
*Material not tested; estimated value based on known material composition and/or miscellaneous literature data.
The Radiation Index (RI) is defined as the logarithm, base 10, of the absorbed dose in Mega grays at which the flexural stress/strain at break of the tested material is reduced to 50% of its original value, under specified conditions of irradiation. The most radiation-sensitivie of these two properties is chosen as the reference critical property.
-

Although it is common market practice to pre-assess only resins for biocompatibility, this fails to consider how the conversion process may impact biological safety.
For our Life Science Grade materials, we conduct an additional level of pre-assessment at the stock shape level, giving you biological safety data that is one step closer to the final component.
Explore the biocompatibility pre-assessment table for detailed compliance information for LSG products.

Depending on your specific application within the Healthcare, Life Science, or Bioprocessing field, the materials used must meet a range of stringent regulatory requirements.
To support you in successfully navigating your product journey, we pre-assess the Life Science Grades on both the resin and stock shape levels. This allows us to present you with intended uses for each material grade, reducing time and resources spent on material selection and in regulatory approval later on.

The new EU MDR involves major changes that will impact the way that medical device manufacturers approach the material selection process.
In this webinar, held in November 2023, three experts walk you through the background of the new MDR, its implications for the polymeric material sourcing process, and measures that device manufacturers can take to prepare.

Our offering for customers in healthcare
Applying relentless attention to technical and regulatory compliance, Mitsubishi Chemical Group offers a full spectrum of thermoplastic solutions for the healthcare industry.
MatFind
Quickly find the optimal engineering plastic for your application. Simply input the performance properties you require, and MatFind will help you filter and compare materials.
Implantable polymers for orthopedics
Part of Mitsubishi Chemical Group's Advanced Materials Division, MediTECH™ is the market's largest portfolio of pre-assessed, implantable PEEK and UHMW-PE stock shapes.
Looking for something specific? Get in touch with our specialist teams by filling in the contact form. We’re ready to meet your next challenge.
Contact us
